Exp 14b: Heat & Thermo Lab 2
Temperature and Heat Transfer
Ever notice how some foods stay hotter longer than
others? Boiled onions or pizza may
burn your tongue if you try to eat it too quickly. But mash potatoes or toast may be eaten straight out of the
stove.While it may take 15 minutes to heat room temperature water to boiling,
it will take only 2 minutes to heat the same mass of iron to the same
temperature, and less than a minute for silver. As you know by now, changing somethingÕs temperature means
changing the average kinetic energy per molecule in that substance. In other words, heat is just another
form of energy. In fact: heat
is defined to be energy that is transferred from one place to another because
of a difference of temperature. Futhermore, the amount of heat (energy)
required to raise the temperature of a given sample of a particular substance by one degree Celcius is
called itÕs Heat Capacity, © of that particular sample of the
substance. Specific Heat,
(denoted by lower case ÒcÓ) on the other
hand, is defined to be the amount of heat
(energy) required to raise the temperture of 1 gram of that substance by 1û C. In
other words, SPECIFIC HEAT is heat capacity per unit mass of the substance.
ItÕs the ÒcÓ in the formula Q = mcDT. The introduction of this formula is the primary purpose of this lab.
Lab GroupNames
Author:________________________
Technician:____________________________
Analyst:_________________________________
Date: ________________________
The objective of lab 2 is to introduce you to two
ideas. How much temperature
changes when energy is transferred to something (like banging on the penny)
depends upon two things:
ItÕs mass and itÕs specific heat capacity.
Activity 1. Convincing oneself that electricity really can generate
heat.
WeÕre going to use electricity to deliver chunks of energy so the first thing we need to do is convince you that electricity really does deliver heat. For this observation you will need the following:
á A Genecon hand-operated generator - Cab 1E
á Alligator clip leads for the generator
á A miniature light bulb and socket - Cab 1D
á An 18Ó (or so) length of nichrome wire - In Genecon box
á A finger
á A lab partner (need a volunteer)
á 8Volts DC (approximately) from red & black plugs under the table
a) Connect the Gencon to the light bulb and turn the crank. Does the light bulb light? Now disconnect it and turn the crank. Describe the difference in cranking before and after disconnecting the bulb.
b) Now wrap the wire loosely around your index finger, attach it to the generator and have your partner turn the crank. Do you feel the heat?
Objective 1: Determining the relationship between heat added (Q) and temperature change (DT). In other words, proving that Qin = mass x heat capacity x Temp change = m c DT. Power P=VI in J/s. So P Dt will be energy in Joules, i. e., Qin = VI Dt . Notice that V and I will be constants in this formula, so if we double the time we double the heat in, halving the time halves the heat in and so forth.
Note: There
is a lot of stuff we are using here we havenÕt covered yet. There are two basic units of heat used in this
course, calories and joules. They
are related by the formula 1 cal = 4.186J. So the division by 4.186 in the table below is to convert the energy units to
calories so that the specific heat of
water will be one calorie/Co/g.
Activity 2: Adding the same amount of heat to different masses of water.
Apparatus: Labpro
& temp probe, in the red tool box, voltmeter & ampmeter, G/3 C, heating
coil, cab 4 D, wires, back wall pegboard.SPST, cab 1C, 10 V DC, under table.
A. WeÕre
going to have 4 setup groups, each with a different mass of water. WeÕll put the same amount of heat in
each chunk of water. WeÕll obtain
a Temperature (T) vs time (t) curve using the Standard Temperature Probe (under
the Apple Menu). With a little
help from Excel or Graphical Analysis weÕll obtain a Q vs DT curve and see what
it tells us.
Group 1: place 125 g of room temperature water in a styrofoam cup inside the plastic cup.
Group 2: Place 175 g room temperature water in a styrofoam cup inside the plastic cup
Group 3: place 200 g of room temperature water in a styrofoam cup inside the plastic cup.
Group 4: place 290 g of room temperature water in the plastic cup.
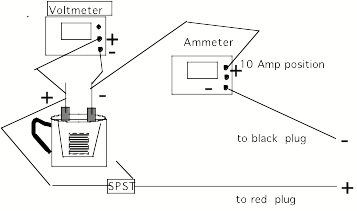
All groups:
1. Hook up the apparatus as indicated in the diagram below.
2. Set the temperature scale to Temperature 1.
3. Set the time scale to 210 s, and the temperature scale to 0 to 100.
Do not throw the switch until the instructor examines your setup.
4. Important! Close the switch and start collecting simultaneously! Heat the water for 210 s. (change the time scale) Stir Constantly!!
5. One person must record the voltage V and the current I every 25 seconds.
6. After your
time runs out, open the switch. Under Analyze, select Examine and
obtain 8 values of T and t to
place in a table with headings indicated below in a Graphical Analysis
worksheet . Use the
average of your voltage and current values for V and I in the formula for Q in the table.
|
I |
V |
Mass |
t |
Dt=t-to |
T |
DT=T-To |
Q = V*I*Dt (J) 4.186(J/cal) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5. Obtain a Q vs DT
graph including line of best fit and equation of the line. Use this and information obtained from
the other two groups to fill in the chart below.
|
Group |
Mass |
Slope of Q vs DT |
% difference btwn m and slope |
|
1 |
125 g |
|
|
|
2 |
175 g |
|
|
|
3 |
200 g |
|
|
|
4 |
250 g |
|
|
6. Include a print out of your Q vs DT data table and graph at this
location in your lab writeup.
Does the slope turns out to be
very close to the mass of your water sample? (It should.)
B. Finding the specific heat of antifreeze. Repeat A steps 1 - 4 with Antifreeze.
|
I |
V |
Mass |
t |
Dt=t-to |
T |
DT=T-To |
Q = V*I*Dt (J) 4.186(J/cal) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5. Fill in the chart below. Note that if Q = mcDT
and slope = k = mc , then c = ?.
|
Group |
Mass |
Slope k of Q vs DT |
c
= k/m |
|
1 |
125 g |
|
|
|
2 |
175 g |
|
|
|
3 |
200 g |
|
|
|
4 |
250 g |
|
|
7. Discussion:
Notice that the antifreeze gains more temperature than the water with
the same amount of heat added.
That is because it has less capacity to absorb heat without affecting its temperature than
water. This c = k/m is
antifreeze's ability to absorb heat as a fractional part of water's ability to
absorb heat per unit mass per
centigrade degree where heat is
in calories.
9. Question: Based upon the information of part
A,Step 6, what must be the specific heat of water according to your data? What is your percent error ?
C. (Optional for 5 points bonus). Repeat B 1 - 6 to find the specific heat of cooking oil.
|
I |
V |
Mass |
t |
Dt=t-to |
T |
DT=T-To |
Q = V*I*Dt (J) 4.186(J/cal) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Group |
Mass |
Slope k of Q vs DT |
c = k/m |
|
1 |
125 g |
|
|
|
2 |
175 g |
|
|
|
3 |
200 g |
|
|
|
4 |
250 g |
|
|
11.
Determine the specific heat of oil.
Problems for
Lab 2: (3 Points)
1. How much heat (in calories) is required to raise the
temperature of 400 g of copper by 60û ?
Look
up the specific heat of copper in your textbook.
2. If 3000 calories of energy raises the temperature of a
substance from 40û C to 55û C, what is it's specific heat capacity?
3. 1500 calories is added to alcohol at 20ûC. Its final temperature is 40û C. What is it's mass?