Workbook Chapter 10
17.9 Ideal Gas Law: PV = nRT
R = 8.31J/mole.K = .08205 atm l/(mole K) Note: T must be in
Kelvins for all this stuff!
k=R/NA = 1.38x10-23 J/K
NA = 6.02x1023
MN2 = molecular mass of nitrogen gas = 28 g/mole
n = number of moles = Mass / Molecular wt of substance = m/M
1 atm = 1.013x105N/m2 = 1 Pascal
Note the alternate form of Ideal Gas Law: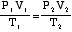
Chapter 19: First Law of Thermodynamics:
Total Energy of the system DU
= Q-W and W =ÝPdV or
DU = Heat gained by the
system - Work done by the system
= Energy gained by heat + Energy gained due to work
Heat added: Q > 0
Heat lost: Q < 0
Work done by system DV >0 W > 0
Work done on system DV <0 W < 0
Adiabatic Process: Q = 0 => DU = -W
Cyclic Process: DU = 0 Þ Q = W
Isobaraic Process: DP = 0 Þ W =
PDV
Isovolumetric Process: DV = 0 Þ W
= 0 Þ DU = Q
Isothermal Process: DT = 0 Þ
DU = 0 Þ Q = W =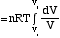
Boiling Process: DU = Q-W = mLV
- P(VV - VL)
Chapter 18 Notes on Kinetic Theory of
Gases
P= (2/3(N/V)(1/2)(mvavg2) so
PV=(2/3)N(1/2)(mvavg2) = NkT so
T =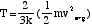 (This is a RBD!)
(This is a RBD!)
Average Kinetic Energy of a molecule (1/2)
mvavg2 = (3/2)kT, But vx2
= (1/3) vavg2 so
(1/2) mvx2 = (1/2)kT or vx
avg2 = kT/m and
vavg2 = 3kT/m where m =
mass of 1 molecule of the gas Total Energy U of a system of an
Ideal Gas
E = N(1/2)(m…v2) = (3/2)NkT = (3/2)nRT so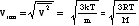
U = (3/2)NkT = (3/2)nRT (monatomic Gas)
Isovolumetric Process DU = Q =
(3/2)nRDT(monatomic Gas)
Molar Specific Heat Q = nCv DT
= (3/2)nRDT, or, Cv = (3/2)R =
2.98(cal/K.mole) = 12.5(J/k.mole) for all monatomic gases, so U =
nCvT for constant volume, or U = nCpT for
constant pressure problems.
For non-monatomic gases, Q=nCpDT
(isobaric processes)
Q=nCvDT )isovolumetric
processes)
Cp-Cv = R for all ideal gases R = 1.99
cal/(Mole.K)
For all monatomic gases Cp= 4.98 cal/(Mole.K)
Cv= 2.98cal/(Mole.K) and
Cp/Cv = 1.67
Adiabatic Process (no Heat exchanged with surroundings)
PV = constant so ToVo - =
TfVf -
Sum-up:
Heat: energy transferred from one object to another because of
a temperature difference.
Temperature: The average kinetic energy of the molecules in
an object.
Internal (mechanical) energy: The total kinetic energy of all
the molecules in an object.
Chapter 22 Notes: Laws of Thermodynamics
First Law of Thermo: Total Energy of the system U =
Q-W
Heat Engines: In1 cycle , DU = 0 so W =
Qnet = Qh - Qc
Qh - Qc
efficiency (eff) = --------------
Qh
Carnot Engine: Ideal Heat Engine, maximum efficiency of any heat
engine is that of a carnot engine.
efficiency of a carnot engine (effc) = 1 -
(Tc/Th) Where Tc is the temperature
of the cold source and Th is the temperature of the hot source in
Kelvins.
Coefficient of Performance for Heat Pumps (and air conditioners)
COP (heating) = QH/W ,
COP(cooling) = QC/W,
Otto Cycle: eff = 1 - 1/(V1/V2)g-1
Expansion 10 point Bonus Problem.
A construction worker uses a steel tape to measure the length of an
aluminum support column. If the measured length is 18.7 m when the
temperature is 21.2 ° C, what is the measured length when the
temperature rises to 29.4° C? (Note: Do NOT neglect the
expansion of the steel tape.)